
Keeping a regular medication routine is one of the most important parts of managing a chronic condition. Studies show that following a prescribed dosing schedule helps maintain control of inflammation and lowers the chance of relapse. Missing or delaying doses, on the other hand, can make treatment less effective over time.
This is especially true for people taking Entyvio (vedolizumab), a biologic therapy used to treat ulcerative colitis and Crohn’s disease. Entyvio works best when given on a consistent schedule that allows the body to build and maintain steady drug levels. Treatment begins with an induction phase, where doses are given more frequently to establish response, followed by a maintenance phase to keep symptoms in remission.
In this article, we’ll explain how the Entyvio dose schedule works, describe what to expect during each phase, and share practical ways to stay on track with treatment for long-term disease control.
Key Takeaways
- Entyvio (vedolizumab) is used for ulcerative colitis and Crohn’s disease, with dosing divided into two phases: an induction phase to establish therapeutic levels and a maintenance phase to sustain remission.
- The IV induction schedule includes 300 mg infusions at Weeks 0, 2, and 6, each administered over approximately 30 minutes.
- After induction, maintenance therapy continues with 300 mg IV every 8 weeks; some patients with reduced response may benefit from dose intensification every 4 weeks under supervision.
- The subcutaneous (SC) Entyvio Pen offers a convenient at-home option. The first injection is given one week after the Week 6 IV infusion (at Week 7), followed by 108 mg every 2 weeks.
- Both IV and SC formulations show comparable safety, efficacy, and drug exposure, allowing clinicians to personalize therapy based on preference and disease stability.
- Missed doses should be taken as soon as possible, then return to the regular schedule; patients should never self-adjust dosing without medical guidance.
- Regular lab monitoring and safety assessments help detect infections, infusion reactions, or liver changes early.
About: Doctor Medica is your trusted supplier of top-quality dermal fillers, viscosupplements, and more for your medical practice. We offer genuine products from leading brands at the lowest prices in the market. If you’re looking to order Entyvio online for your practice, contact the Doctor Medica today.
Induction Dose Schedule for Entyvio

The induction phase helps build therapeutic levels of vedolizumab, the active ingredient in Entyvio, within the body. During this stage, treatment focuses on reducing gut inflammation and helping patients experience early relief of symptoms such as stool urgency, bleeding, and abdominal discomfort.
The standard intravenous (IV) induction schedule for Entyvio is as follows:
- Week 0: First 300 mg IV infusion, administered over approximately 30 minutes.
- Week 2: Second 300 mg infusion.
- Week 6: Third 300 mg infusion.
This three-dose sequence helps saturate α4β7 integrin receptors in the gastrointestinal tract, allowing vedolizumab to block lymphocyte trafficking that drives inflammation. By Weeks 10 to 14, many patients report meaningful improvement in bowel symptoms and overall comfort.
During this period, healthcare professionals closely monitor for any infusion reactions, though these are generally mild and short-lived. Regular follow-ups help clinicians determine whether the patient is responding well before moving to the maintenance phase. For detailed clinical guidance, practitioners can refer to the official Entyvio prescribing information, which outlines dosing protocols and safety monitoring recommendations.
Maintenance Dosing for Entyvio IV
Once the induction phase is complete, patients enter the maintenance phase, which helps sustain remission and prevent flare-ups. The standard Entyvio IV maintenance dose is 300 mg every 8 weeks. This schedule provides consistent drug levels and predictable symptom control for most patients.
Core Aspects of IV Maintenance Therapy
- Long-term monitoring: Regular lab testing, stool markers, and periodic colonoscopies help track treatment response.
- Consistency: Missing or delaying infusions can lead to reduced drug levels and symptom recurrence.
- Clinic-based supervision: Infusions are administered in a controlled setting to manage potential hypersensitivity reactions.
In some instances, patients who experience a partial or declining response may require dose intensification, shortening the interval to every 4 weeks. This strategy can restore therapeutic levels and improve remission rates, particularly in more aggressive cases of Crohn’s disease.
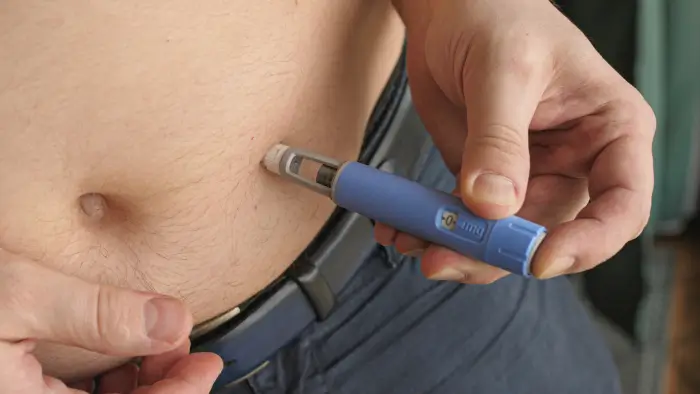
Subcutaneous Maintenance Option (Entyvio Pen)
For patients who prefer home administration or wish to reduce clinic visits, the Entyvio Pen provides a subcutaneous (SC) vedolizumab option with safety and efficacy similar to the IV formulation. This alternative allows for greater independence while maintaining steady control.
The Entyvio Pen schedule is:
- First SC injection: Administered one week after the Week 6 IV infusion (at Week 7).
- Maintenance: Continue with 108 mg SC injection every 2 weeks.
Each prefilled pen delivers the dose into the abdomen or thigh in just a few seconds. Studies have shown that SC dosing achieves comparable drug exposure and clinical outcomes to the IV formulation.
Entyvio Pen Advantages
- Fewer clinic visits and more flexibility for long-term management.
- Consistent serum concentrations, helping maintain steady disease control.
- Improved treatment adherence and patient comfort.
Patients transitioning from IV to SC dosing should receive training on proper injection technique, safe storage, and missed dose protocols. If they miss a dose, take it as soon as possible, then resume the regular 2-week schedule.
Special Considerations & Off-Schedule Adjustments
While the Entyvio dosing schedule is structured, treatment plans may need to be adjusted to accommodate real-world challenges, such as missed appointments or fluctuating disease activity. Clinicians tailor recovery or reloading schedules to maintain disease control and prevent relapse.
- Missed Dose Management: Administer the missed dose as soon as possible, then return to the regular dosing schedule.
- Dose Intensification: For patients losing response on standard maintenance, the IV infusion interval may be shortened to every 4 weeks under medical supervision.
- Switching Formulations: Patients may transition between IV and SC dosing based on response, convenience, and physician guidance.
- Laboratory Monitoring: Routine liver function tests and infection screenings are recommended throughout therapy.
- Safety Checks: Patients are evaluated for potential hypersensitivity or infection risks before and after each infusion.
Patients should never adjust their own dosing schedule without consulting their healthcare provider. Ongoing communication between patients and clinicians ensures that any changes maintain both clinical effectiveness and treatment safety.
Conclusion
Understanding the Entyvio dose schedule helps patients and healthcare providers work together toward lasting remission. Whether administered through IV infusion or the subcutaneous Entyvio Pen, staying consistent with scheduled doses is the foundation of successful treatment.
The flexibility between both forms allows for a personalized approach based on patient preference, disease activity, and lifestyle needs. Missing or delaying doses without medical advice can reduce treatment efficacy, so maintaining regular follow-up and clear communication is key to long-term success with vedolizumab therapy.
FAQs
1. How often do I receive Entyvio infusions?
During induction, patients receive Entyvio at Weeks 0, 2, and 6. Afterward, the maintenance phase continues every 8 weeks. However, your doctor may recommend a shorter interval for better response.
2. Can I switch from Entyvio IV to the Entyvio Pen?
Yes. Once stable on IV therapy, many patients transition to the subcutaneous Entyvio Pen, which is administered every 2 weeks at home.
3. What happens if I miss an Entyvio dose?
Take the dose as soon as possible, then return to your regular schedule. Inform your provider if symptoms worsen or if several doses are missed.
4. Is dose intensification safe with Entyvio?
Yes, when done under medical supervision. Shortening infusion intervals (to every 4–6 weeks) can help patients who lose response over time regain remission.
References
Entyvio (vedolizumab) [package insert]. Takeda Pharmaceuticals U.S.A., Inc; 2022. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/125476Orig1s046lbl.pdf.
ENTYVIO® (vedolizumab) Official HCP Site. https://www.entyviohcp.com/dosing-administration
Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. New England Journal of Medicine. 2013;369(8):699-710. doi:10.1056/nejmoa1215734
Related Articles
Joanna Carr
Understanding the Value of Filorga Injections: Balancing Cost and Benefits
Discover the value of Filorga injections at Doctor Medica. Learn how to balance cost and benefits for optimal results and satisfaction.
Joanna Carr
Jaydess and Hair Loss – Are They Related?
Jaydess is a hormonal intrauterine device (IUD) used for contraception, releasing the hormone levonorgestrel.
Joanna Carr
Euflexxa Generic Name – About Sodium Hyaluronate
Learn about sodium hyaluronate, the generic name for Euflexxa. Discover how this naturally occurring substance mimics synovial fluid to relieve knee o...


