
Viscosupplementation, a treatment that involves injecting hyaluronic acid (HA) into knee joints, has become increasingly popular as a non-surgical approach for managing osteoarthritis pain. According to a 2022 systematic review, HA injections offer modest but clinically meaningful improvements in pain relief and joint function compared to placebo, with therapeutic effects lasting up to 26 weeks. These findings have accelerated interest in HA therapies as viable alternatives to invasive procedures.
Among the numerous viscosupplements available, Euflexxa has emerged prominently due to its innovative, non-avian, bioengineered formulation containing sodium hyaluronate gel. Designed to restore joint lubrication and enhance shock absorption, Euflexxa aims to alleviate osteoarthritic symptoms and improve patients’ quality of life. Although HA treatments are not suitable for every patient, Euflexxa is recognized for its potential to enhance mobility and possibly postpone the need for surgical intervention.
In this article, we’ll closely examine and compare Euflexxa vs Synvisc, highlighting essential differences in their formulations, efficacy, methods of administration, and their overall impact on patient outcomes.
Key Takeaways
- Euflexxa is a non-avian, bacterial fermentation-derived viscosupplement, offering lower allergy risk and gradual, sustained pain relief.
- Synvisc-One is an avian-derived, cross-linked hyaluronic acid product, administered as a convenient single injection, typically providing symptom relief beginning around four weeks.
- The standard dosage for Euflexxa consists of three weekly injections (2 mL each), making it ideal for patients preferring incremental improvement and careful monitoring between doses.
- The choice between Euflexxa and Synvisc depends significantly on individual factors, including patient allergy history, the severity of osteoarthritis, overall health status, and lifestyle preferences.
- Both products offer clinical effectiveness for up to six months. Individual patient responses can vary considerably based on age, severity, and overall health.
- Euflexxa’s non-animal origin makes it particularly suitable for patients with allergies or autoimmune conditions, whereas Synvisc-One’s single-dose regimen suits those prioritizing fewer clinical visits.
- Understanding each product’s formulation, administration schedule, and safety profile ensures optimal treatment decisions tailored to individual patient needs.
About: Doctor Medica is your trusted supplier of top-quality dermal fillers, viscosupplements, and more for your medical practice. We offer genuine products from leading brands at the lowest prices in the market. If you’re looking to order Euflexxa online for your practice, contact Doctor Medica today.
Origin and Molecular Profile
Euflexxa is a non-avian sodium hyaluronate derived from bacterial fermentation with a molecular weight (MW) of approximately 2.4 million daltons (MDa). Its single-chain molecular structure ensures smooth viscosity, closely mimicking natural joint fluid. Additionally, its bacterial origin substantially reduces the risk of allergic reactions associated with animal proteins, providing a safer profile for patients sensitive to avian components.
In contrast, Synvisc is derived from avian sources, containing cross-linked hyaluronic acid (HA) molecules with significantly higher molecular weights—around 6 MDa for Synvisc-One. The cross-linking creates a thicker, more viscous gel that extends joint residence time, enhancing shock absorption and lubrication compared to linear HA formulations.
These distinct molecular profiles influence each product’s therapeutic approach: Euflexxa aims to closely replicate the body’s natural joint fluid viscosity, while Synvisc prioritizes extended joint retention and robust cushioning.
Regimen and Dosage Differences
Euflexxa follows a structured three-injection protocol, with each injection containing 2 mL administered weekly. This multi-dose regimen allows healthcare providers to monitor patient progress between injections, adapting care based on individual responses. Additionally, the recommended Euflexxa dosage schedule supports gradual symptom improvement, typically preferred by patients seeking steady, predictable relief.
Conversely, Synvisc-One offers convenience through a single 6 mL injection, appealing to individuals who prefer fewer clinic visits. Traditional Synvisc variants also involve three injections but at higher volumes per injection, providing flexibility depending on clinical requirements.
Choosing between multiple smaller injections (Euflexxa) and a single high-volume injection (Synvisc-One) significantly depends on patient preferences, convenience, adherence potential, and scheduling constraints, all of which impact treatment success.
Immunogenicity and Allergy Risk
The risk of immunogenic reactions, or the immune system responding adversely to injected substances, is an important consideration when selecting a viscosupplement for knee osteoarthritis. The differences in molecular origin between Euflexxa and Synvisc have a significant impact on their respective allergy profiles.
Euflexxa: Non-Avian, Reduced Immunogenicity
Euflexxa is manufactured through bacterial fermentation, meaning it contains no animal-derived proteins. This formulation significantly reduces the risk of immune-mediated side effects, making it particularly suitable for patients who are sensitive to animal proteins or have a history of allergic reactions.
- Origin: Non-avian, bacterial fermentation-derived.
- Allergy Risk: Significantly lower due to the absence of animal proteins.
- Common Reactions: Mild, temporary injection-site reactions such as redness, slight swelling, or mild discomfort.
- Reaction Duration: Typically resolves within 1–2 days without medical intervention.
Because of its minimal allergenic potential, Euflexxa offers reassurance for patients with:
- Known allergies or sensitivities to animal-based components.
- Compromised immune systems.
- Autoimmune disorders.
Synvisc: Avian-Derived, Higher Immunogenic Potential
In contrast, Synvisc is formulated from avian (bird-derived) proteins and contains cross-linked hyaluronic acid (HA) molecules. This avian origin inherently carries a greater risk for immune responses, especially in individuals with sensitivities to birds, poultry, feathers, or eggs.
- Origin: Avian-derived, containing bird proteins.
- Allergy Risk: Higher potential for allergic responses, especially in bird or egg-sensitive patients.
- Common Reactions: Increased likelihood of injection-site swelling, stiffness, warmth, redness, or localized inflammation.
- Reaction Severity: Usually mild but may be more pronounced in allergic or immunocompromised patients.
Due to its composition, clinicians should carefully screen patients before treatment with Synvisc, particularly those who:
- Have documented allergies to avian proteins, eggs, poultry, or feathers.
- Have autoimmune conditions that predispose them to exaggerated immune responses.
- Have a history of severe or frequent allergic reactions.
Patient Selection Considerations
Ultimately, individual patient evaluation is critical:
- Patients with a known or suspected avian allergy typically benefit from the safer profile of Euflexxa.
- Patients without sensitivities who require fewer injections or have scheduling constraints may tolerate Synvisc comfortably.
Healthcare providers should always thoroughly review a patient’s allergy history, health status, and preferences before making a final treatment recommendation.
Clinical Efficacy and Duration
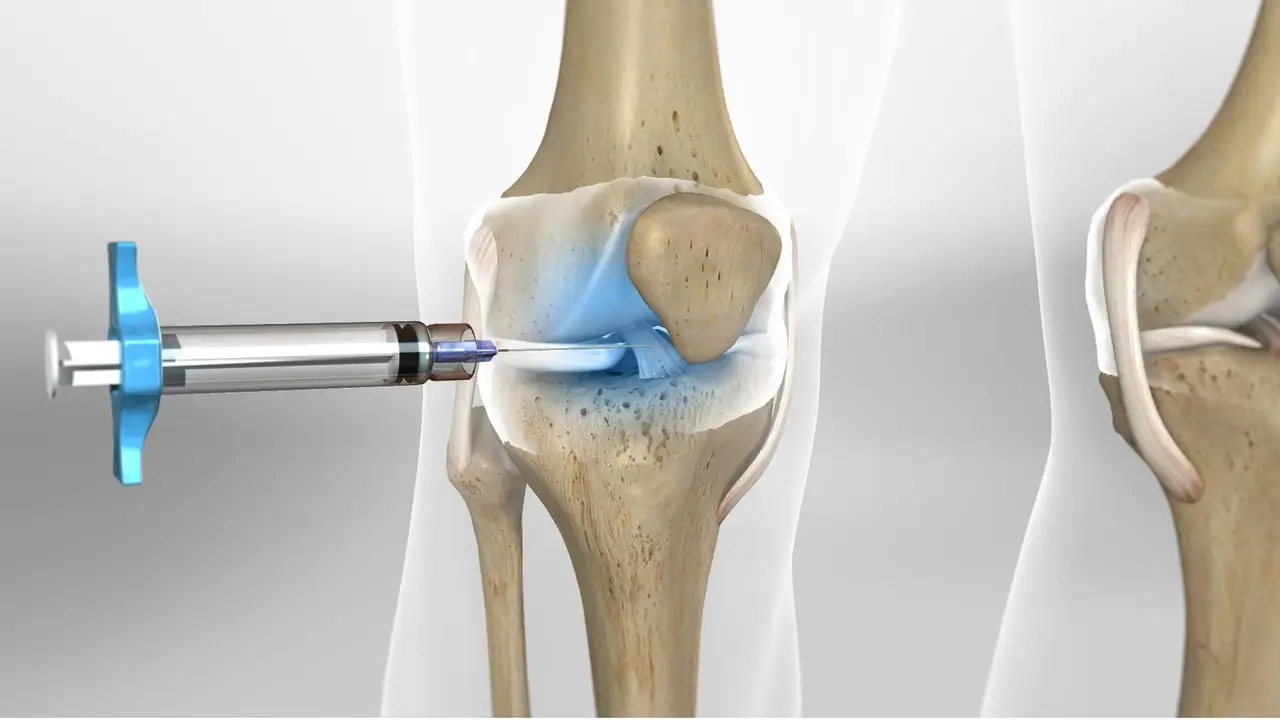
Understanding the onset and duration of action of each viscosupplement is essential for informed treatment decisions. Both Euflexxa and Synvisc-One demonstrate clinical efficacy in relieving osteoarthritis-related knee pain and improving joint function. However, their timelines differ and can be influenced significantly by patient-specific variables such as age, overall health status, and osteoarthritis severity.
Below is a detailed comparison outlining clinical expectations for each treatment:
| Feature | Euflexxa | Synvisc-One |
| Onset of Pain Relief | Gradual, noticeable improvement often after the second or third injection. | Typically begins around four weeks post-injection, though some patients report earlier relief. |
| Time to Peak Effect | Approximately 6 weeks after treatment initiation. | Commonly between 4 to 6 weeks post-injection. |
| Duration of Relief | Lasts up to 6 months; some patients report benefits extending up to 9 months. | Approximately 6 months, though duration varies by patient. |
| Injection Schedule | Three weekly injections (2 mL each). | One-time, single injection (6 mL). |
| Re-treatment Eligibility | Typically every 6 months, or earlier based on symptom recurrence. | Repeatable every 6 months if clinically necessary |
| Clinical Trial Support | Robust data demonstrating consistent efficacy and safety across various patient demographics. | Long-established clinical support, especially effective for patients needing more immediate symptom relief. |
Patient responses vary significantly, and the described timelines should be viewed as general guidelines rather than guaranteed outcomes. Personalized patient evaluation ensures optimal alignment between clinical expectations and treatment choice.
Safety and Adverse Event Comparison
Considering potential side effects is vital when selecting treatments for knee osteoarthritis. Both Euflexxa and Synvisc exhibit strong safety profiles, widely accepted in clinical practice. However, molecular differences influence their respective side effect profiles, with patient-specific factors—such as disease severity, health conditions, and allergic histories—playing essential roles in the likelihood and intensity of adverse events.
Common side effects associated with both treatments include:
- Injection Site Discomfort: Mild aching or pain post-injection, typically subsiding within 24–48 hours.
- Joint Swelling and Warmth: Slightly more prevalent with Synvisc’s cross-linked gel, manageable with ice and rest.
- Temporary Joint Stiffness: Usually mild and resolves quickly without intervention.
- Localized Redness or Irritation: Generally short-lived, rarely requiring medical attention.
Additionally, systemic reactions—though uncommon—are more plausible with Synvisc due to its avian-derived proteins. Conversely, Euflexxa’s bacterial fermentation reduces systemic immunological reactions, providing reassurance for patients who are sensitive to other products.
Clinicians should emphasize that individual reactions vary, reinforcing personalized medical evaluations to ensure both safety and patient satisfaction.
Conclusion
In comparing Euflexxa vs Synvisc, both viscosupplements offer reliable, drug-free treatment options for managing osteoarthritis knee pain. Euflexxa, with its structured three-injection protocol, provides gradual relief and minimal allergic risk through its non-avian formulation. Alternatively, Synvisc-One appeals to patients seeking fewer injections, convenience, and potentially quicker relief onset, despite slightly higher immunogenicity.
Ultimately, the optimal choice depends on patient-specific considerations, including individual medical history, allergy risk, desired treatment frequency, symptom severity, and logistical constraints. Careful consultation between patients and healthcare providers is crucial to determine the most effective therapeutic match for achieving lasting relief and an improved quality of life.
FAQs
1. How many Euflexxa injections do I need?
The standard dosage schedule consists of three injections, administered one per week, in a clinical setting.
2. How does Synvisc-One compare to Euflexxa?
Synvisc-One offers quicker relief with a high-volume dose. Euflexxa may be a better option for individuals with bird or egg allergies or those who prefer gradual improvement.
3. Can I have both Euflexxa and Synvisc doses?
Generally, physicians choose one product per treatment cycle. Switching may require reassessment, but it is possible under the supervision of a provider.
4. Which product lasts longer?
Both can offer relief for 3–6 months. Synvisc-One’s effects may begin faster; Euflexxa may have a smoother onset and lower allergy risk.
References
Pereira TV, Jüni P, Saadat P, et al. Viscosupplementation for knee osteoarthritis: systematic review and meta-analysis. BMJ. 2022;378:e069722. Published 2022 Jul 6. doi:10.1136/bmj-2022-069722
Maheu E, Rannou F, Reginster JY. Efficacy and safety of hyaluronic acid in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45(4 Suppl):S28-S33. doi:10.1016/j.semarthrit.2015.11.008
Related Articles
Joanna Carr
Depo Provera Side Effects – Common, Rare, Mild, Serious
Common side effects of Depo Provera include weight gain, changes in menstrual cycles, and headaches. Read more on Doctor Medica.
Joanna Carr
Fortelis Vs Juvederm: Similarities And Differences Explained
Interested in learning more about The Similarities And Differences of Fortelis Vs Juvederm Explained? Browse Doctor Medica's comprehensive archive of ...
Joanna Carr
Revolax vs Juvederm – Comparing Dermal FIllers
Revolax lasts up to 24 months because of a unique crosslinking method, while Juvederm uses Vycross technology for lasting effects.


