
Shop with Confidence: Product Authenticity is Guaranteed
All products available at Doctor Medica shop are obtained from respective manufacturers and contain original LOT numbers. Contact us if you have any questions about product LOT numbers.
FAQ
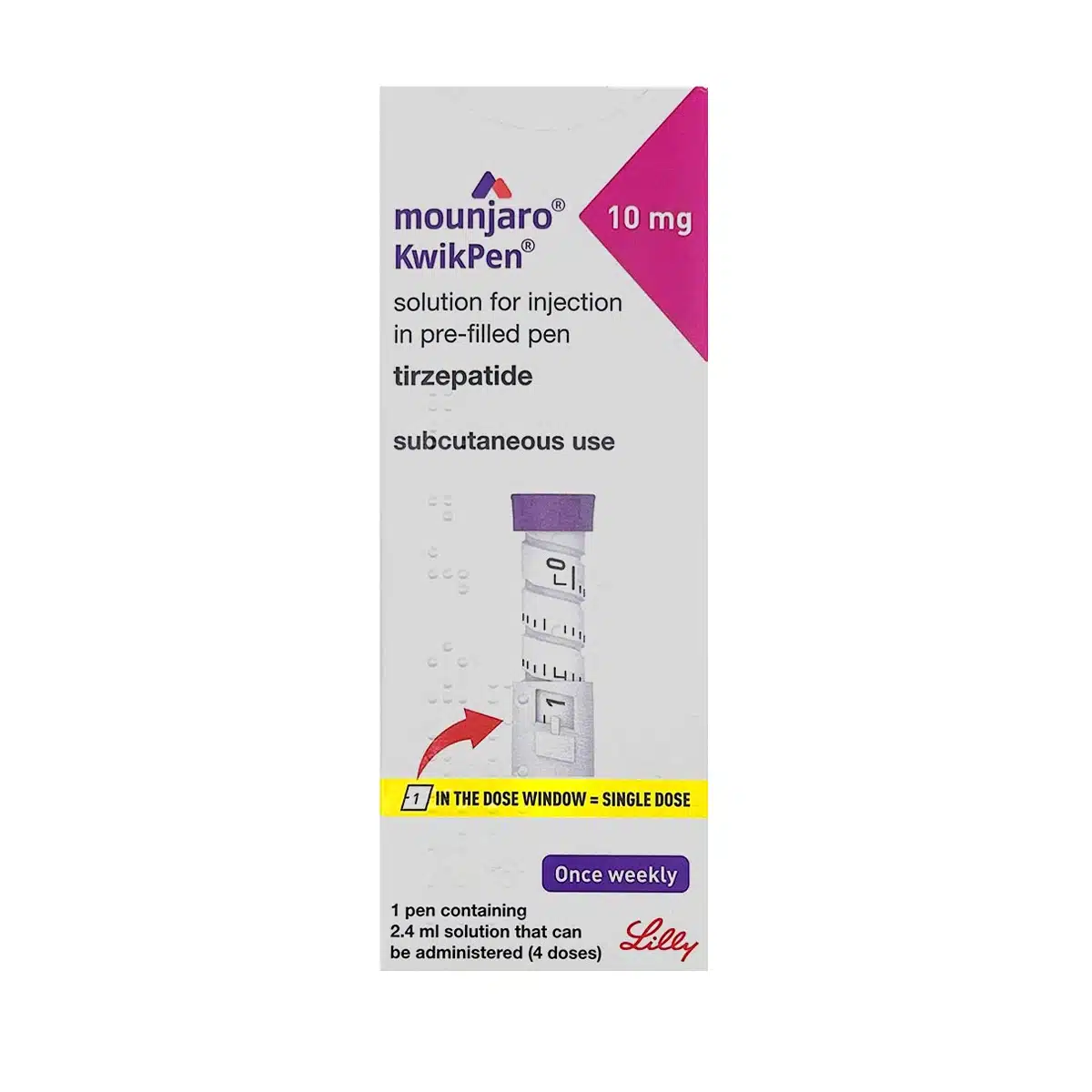
MOUNJARO® 10mg KwikPen® is a prefilled, multi-dose pen containing 10 mg tirzepatide per 0.6 mL injection. The full pen holds 40 mg in 2.4 mL (approx. 16.7 mg/mL) and is designed for weekly subcutaneous dosing. This therapy acts on GIP and GLP-1 receptors and is indicated for both blood sugar management in type 2 diabetes and weight control. The solution is clear, colourless to faint yellow.
-
Injection strength: 10 mg/0.6 mL
-
Pen volume: 40 mg/2.4 mL
-
Concentration: 16.7 mg/mL
-
Mechanism: Dual incretin agonist (GIP & GLP-1)
-
Sites: Stomach, thigh, upper arm
The pen provides a weekly subcutaneous dose, following gradual titration to minimize side effects.
-
Stepwise dosing: Begin with 2.5 mg weekly, escalate every 4 weeks until reaching 10 mg
-
Maintenance options: 5 mg, 10 mg, or 15 mg; 10 mg is a standard maintenance dose
-
Weekly routine: Same day weekly; may adjust if ≥3 days apart
-
Missed injection: If <4 days late, inject promptly; if >4 days, skip
-
Injection: Dial to 1, inject 0.6 mL, hold 5 sec, confirm dose window reset
-
Rotate injection sites to reduce skin irritation
Prescribed for adults with insufficiently controlled type 2 diabetes and for chronic weight management. Always combined with lifestyle interventions.
-
Diabetes care: As single agent or combined with other glucose-lowering therapies
-
Obesity treatment: Adults with BMI ≥30, or ≥27 with comorbid conditions
-
Contraindicated in: Allergy to tirzepatide/excipients
-
Cautions: Not studied in severe GI disease or advanced diabetic eye disease
-
Pregnancy: Discontinue before conception (≥1 month in advance)
-
Typical side effects: GI upset, decreased appetite, occasional hypoglycaemia (esp. with insulin/secretagogues)
Formulated as a clear injectable solution, delivering 10 mg tirzepatide with each 0.6 mL dose.
-
Active drug: Tirzepatide 10 mg
-
Full pen: 40 mg/2.4 mL solution
-
Concentration: 16.7 mg/mL
-
Excipients: Contains benzyl alcohol and buffer components
-
Sodium level: Essentially sodium-free
Each retail unit includes the pen and instructions, but no needles.
-
Pen device: Multi-use KwikPen (4 weekly doses)
-
Documentation: Leaflet + Instructions for Use
-
Needles: Not included
Yes, the most reported effects involve the digestive tract. These are dose-related and generally diminish with time.
-
GI adverse events: Nausea, diarrhoea, constipation; up to 39% incidence at 10 mg
-
Hypoglycaemia: Notable risk if combined with sulphonylurea or insulin
-
Injection-site reactions: ~5% in studies
-
Gallbladder events: Reported but uncommon
-
Rare risks: Acute pancreatitis, hypersensitivity reactions
Proper storage ensures safety and effectiveness. Disposal follows usage schedule.
-
Unopened: Refrigerate at 2–8°C
-
Do not freeze
-
After opening: Keep at ≤30°C, discard after 30 days
-
Each pen: Used for 4 doses, then discard
-
Always remove needle after injection

