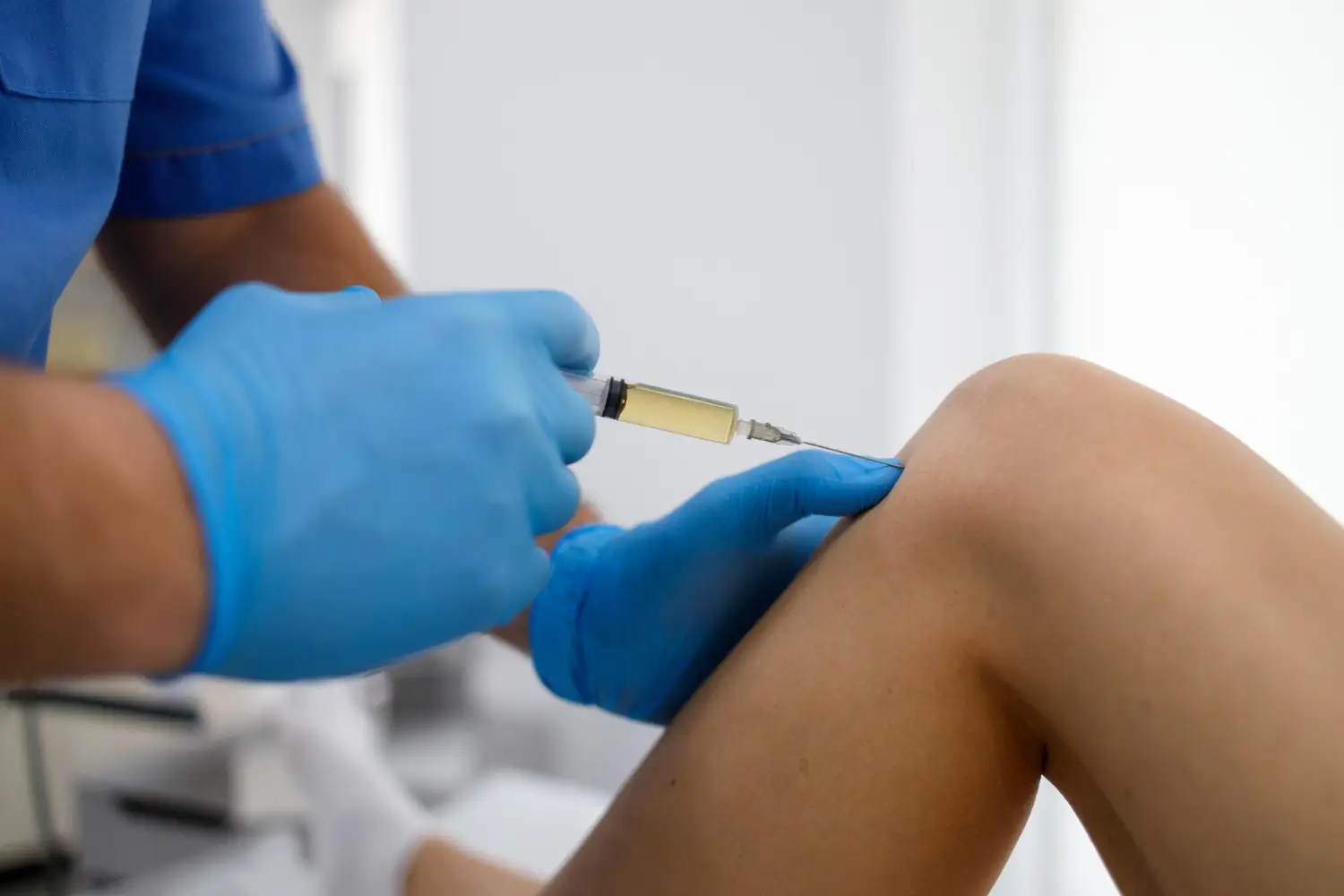
Knee osteoarthritis affects over 14 million people in the United States, making it one of the leading causes of chronic pain and reduced mobility. Managing this condition can be challenging, as patients often struggle to find treatments that provide both long-lasting relief and improved joint function. Viscosupplementation has gained traction as an effective solution for alleviating symptoms and delaying more invasive interventions like surgery.
Durolane stands out for its ability to reduce pain and enhance knee mobility among these treatments. By supplementing the natural hyaluronic acid in the joint, it restores lubrication and cushioning, providing relief from osteoarthritis discomfort and improving patients’ quality of life.
In this article, we will discuss the Durolane FDA approval status, its role in treating knee osteoarthritis, and how it compares with other available treatment options.
Key Takeaways
- Durolane underwent rigorous clinical trials and regulatory evaluations to meet FDA standards, proving its value in treating knee osteoarthritis.
- Using NASHA technology in Durolane offers enhanced joint lubrication and pain relief with a single injection, providing long-lasting effects.
- Clinical trials demonstrated significant pain reduction and improved mobility, with results lasting up to six months.
- FDA approval allows broader use of Durolane by healthcare providers, making it accessible to patients seeking non-surgical osteoarthritis treatments.
About: Doctor Medica is your trusted supplier of top-quality dermal fillers, viscosupplements, and more for your medical practice. We offer genuine products from leading brands at the lowest prices. Contact Doctor Medica today to order Durolane online for your practice.
The Clinical Trials and Regulatory Processes

Durolane underwent rigorous clinical trials and regulatory evaluations to obtain FDA approval, ensuring its safety and efficacy for treating knee osteoarthritis. These trials, conducted by the Durolane manufacturer, Galderma (and later on Bioventus), focused on assessing its ability to reduce pain and improve joint function through its innovative NASHA (Non-Animal Stabilized Hyaluronic Acid) technology.
In pivotal clinical studies, Durolane demonstrated significant pain reduction and mobility improvement compared to placebo and other viscosupplementation treatments. Key findings highlighted its extended duration of relief, with many patients experiencing benefits lasting up to six months after a single injection. The trials also confirmed Durolane’s favorable safety profile, with most side effects limited to mild, temporary injection-site reactions such as swelling or pain.
The regulatory process for Durolane’s approval involved submitting a Premarket Approval (PMA) application to the FDA. The FDA’s Center for Drug Evaluation and Research (CDER) reviewed the clinical trial data, ensuring that Durolane’s benefits outweighed its known risks.
The review included an analysis of the target condition, available treatments, and Durolane’s clinical benefit-risk profile. Key findings from the trials highlighted Durolane’s ability to provide long-lasting pain relief and improved mobility, supporting its approval.
Durolane’s Formulation and Effectiveness

Durolane’s unique, high-viscosity gel is designed to provide superior cushioning and lubrication, reducing friction between joint surfaces and alleviating the discomfort caused by osteoarthritis.
By restoring HA levels in the knee joint, Durolane improves joint function, making movement smoother and less painful. This single-injection treatment delivers targeted relief lasting up to six months, making it a convenient option for patients seeking long-term results.
Clinical studies have demonstrated Durolane’s effectiveness in improving joint function and reducing pain. A single injection of Durolane has been shown to provide significant pain relief and enhanced joint function for up to 26 weeks. Patients report improved mobility and reduced osteoarthritis-related symptoms, making daily activities more manageable.
Insights into the FDA’s Review Process and Regulatory Approval
The FDA’s review process for medical devices like Durolane involves a thorough evaluation of safety, efficacy, and manufacturing quality. This process ensures that the product meets strict standards for its intended use.
Obtaining FDA approval is a significant milestone, as it indicates that a treatment is safe and effective for patient use. For patients and healthcare providers, FDA approval guarantees the product’s reliability and aligns with the highest regulatory standards.
Durolane’s FDA approval underscores its value as a trusted viscosupplement for osteoarthritis management. It validates the safety and effectiveness of its NASHA technology, distinguishing Durolane as a leader among similar treatments. This approval also paves the way for wider adoption by healthcare providers and enhances accessibility for patients seeking long-lasting, non-surgical pain relief.
Conclusion
Durolane’s FDA approval underscores its effectiveness and safety as a viscosupplement for osteoarthritis. Backed by NASHA technology, Durolane delivers long-lasting pain relief and improved joint function with a convenient single-injection protocol. This innovative approach has set new standards in non-surgical osteoarthritis management, benefiting millions of patients.
Its approval not only validates its quality but also ensures wider adoption by healthcare providers, making it a trusted solution for knee osteoarthritis pain.
FAQs
1. What is Durolane used for?
Durolane is an FDA-approved injection treatment for knee osteoarthritis pain. It mimics natural joint fluid, providing cushioning and lubrication to reduce pain and improve mobility.
2. How long does Durolane’s effect last?
A single injection of Durolane can provide relief for up to six months, although individual results may vary.
3. Who manufactures Durolane?
Galderma S.A. initially developed Durolane, which is now managed by Bioventus.
4. Is Durolane safe for everyone?
Durolane is generally safe for most knee osteoarthritis patients. However, it may not be suitable for individuals with joint infections, allergies to hyaluronic acid, or inflammatory joint diseases. Consulting a healthcare provider is essential.
References
Deshpande BR, Katz JN, Solomon DH, et al. The number of persons with symptomatic knee osteoarthritis in the United States: Impact of race/ethnicity, age, sex, and obesity. Arthritis Care Res (Hoboken). 2016 Dec;68(12):1743–1750.
Ernst, D. (2017, September 18). FDA approves Durolane to treat knee osteoarthritis pain. Rheumatology Advisor. https://www.rheumatologyadvisor.com/news/fda-approves-durolane-to-treat-knee-osteoarthritis-pain/
Drugs.com. (2023, August 14). Durolane injection: Uses, side effects & warnings. Retrieved November 18, 2024, from https://www.drugs.com/mtm/durolane-injection.html
Related Articles
Joanna Carr
Amalian Vs Juvederm: Similarities And Differences Reviewed
Interested in learning more about A Review Of The Similarities and Differences of Amalian And Juvederm? Browse Doctor Medica's comprehensive archive o...
Joanna Carr
Durolane FDA Approval Status
Discover the FDA approval status of Durolane, a hyaluronic acid injection for osteoarthritis, and its impact on pain relief and joint health.
Joanna Carr
Artefill Vs Juvederm: Similarities And Differences Explained
Have an interest in learning about The Similarities And Differences of Artefill Vs Juvederm Explained? Browse Doctor Medica's extensive archive of blo...


