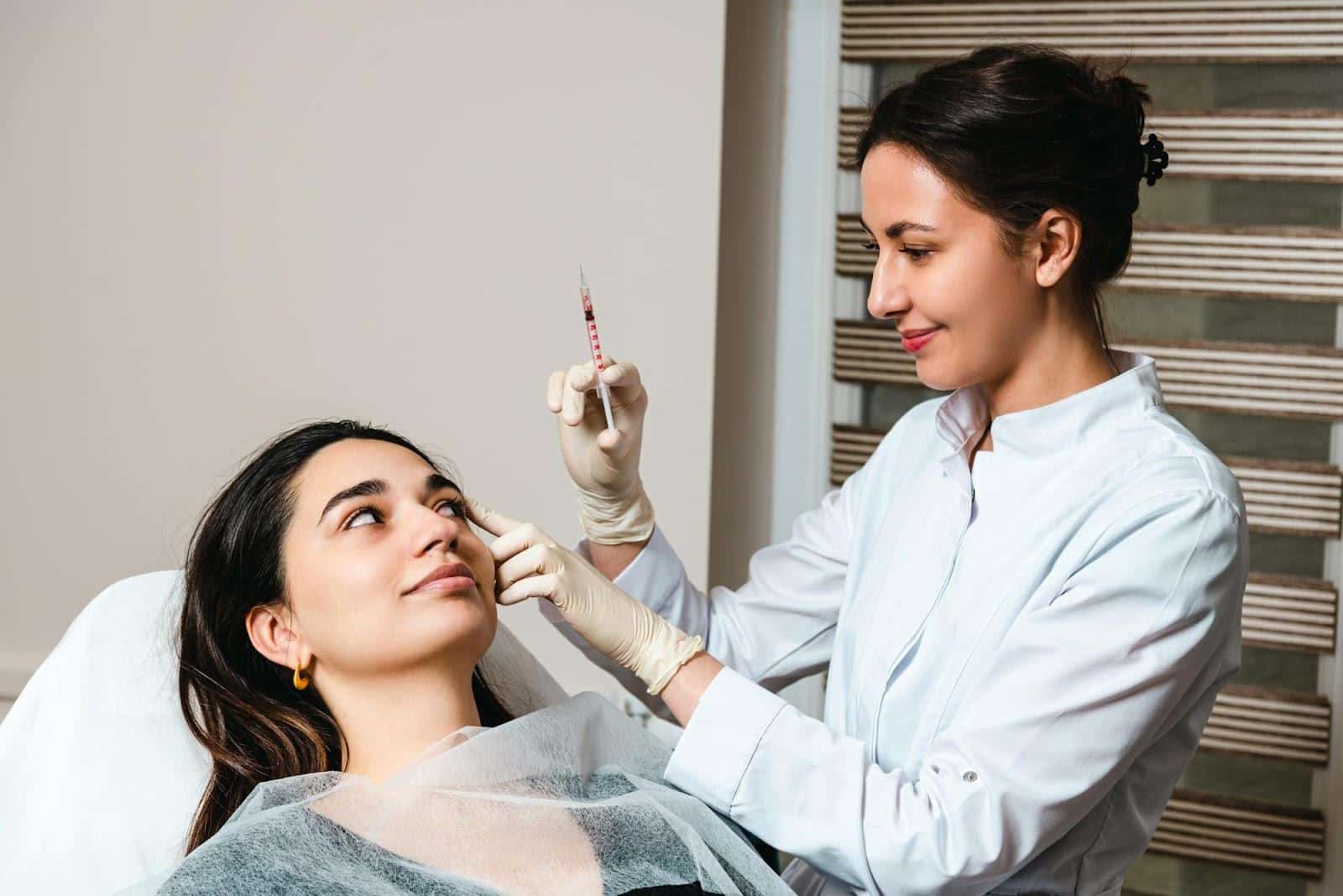
According to the latest report from RealSelf, Dysport has emerged as one of the leading nonsurgical treatments for 2022, boasting a 95% “Worth It” rating. This high satisfaction rate highlights the treatment’s effectiveness, with most patients willing to repeat the procedure.
Dysport is an injectable medication chosen by healthcare professionals for its ability to soften and reduce facial wrinkles. However, its application extends beyond cosmetics, relieving various medical conditions. With Dysport’s increasing popularity, professionals must understand the prescribing information thoroughly for safe and effective use.
In this article, we will explore the essential aspects of Dysport administration so aesthetic specialists and other professionals can deliver optimal treatment outcomes.
Key Takeaways
- Dysport treats wrinkles and muscle tightness by relaxing muscles. It works for frown lines, crow’s feet, and more.
- Before using Dysport, doctors must check patients’ health histories to avoid risks. Reviewing a patient’s medical history includes examining allergies and past treatments.
- Patients with certain medical conditions shouldn’t use Dysport because of severe side effects like trouble breathing or swallowing.
- Doctors must follow the proper dosage and where to inject Dysport for the best results while keeping patients safe from harm.
Introduction to Dysport
Dysport® is a brand name for a prescription medication known generically as abobotulinumtoxinA. It is a neuromuscular blocker that temporarily weakens or paralyzes muscles by blocking the release of a chemical messenger at the nerve endings that tells muscles to contract.
Dysport has two main FDA-approved uses:
- Cosmetic: For aesthetic purposes, healthcare professionals administer Dysport injections to temporarily reduce the appearance of moderate to severe frown lines between the eyebrows (glabellar lines). These injections can also treat other facial areas off-label to deliver facial rejuvenation.
- Therapeutic: Healthcare professionals administer Dysport to treat various medical conditions, such as cervical dystonia, muscle spasms, and stiffness.
Importance of Understanding Prescribing Information for Safe and Effective Use
The prescribing information for Dysport is crucial for ensuring patient safety and treatment efficacy. It includes detailed instructions on dosage, administration, and potential side effects. Understanding these guidelines is essential, as the effects of Dysport can spread beyond the injection site, potentially leading to severe, life-threatening conditions such as difficulties with swallowing and breathing.
This information, typically included in a “package insert,” details essential aspects of the medication. Before administering Dysport, healthcare providers must be well-informed about the patient’s medical history and potential risks.
Indications for Using Dysport
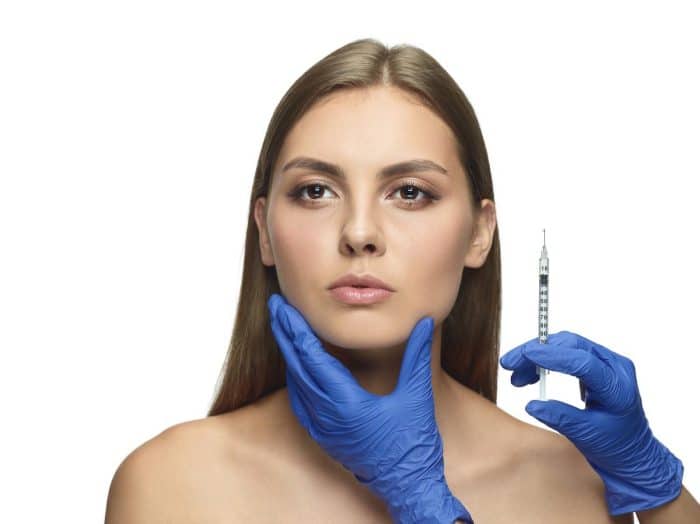
Dysport can treat several conditions and symptoms, cosmetically and therapeutically. An expert can use Dysport to temporarily improve the appearance of moderate to severe frown lines (glabellar lines) between the eyebrows in adults under 65.
These lines form due to repeated muscle contractions during facial expressions like frowning. Dysport injections weaken these muscles, softening the appearance of the lines. Moreover, Dysport can also treat muscle spasticity, a condition characterized by involuntary muscle stiffness and tightness.
Spasticity can occur in various parts of the body due to underlying neurological conditions like cerebral palsy, stroke, or spinal cord injury. Dysport helps manage spasticity by reducing muscle tone and improving flexibility and range of motion.
Specific Areas of Administration
Depending on the patient’s condition, a licensed professional can administer Dysport in various body areas. Dysport is commonly used in cosmetic applications to treat wrinkles in the upper face, such as the forehead, frown lines, and crow’s feet. Doctors can also use Dysport in the lower face and neck to address lines around the mouth, neck bands, and other areas with excessive sweat.
For adults experiencing upper limb spasticity, Dysport injections target specific muscles in the elbow, wrist, and fingers that contribute to stiffness and limited movement. However, Dysport can also treat lower limb spasticity in children two years and older.
A healthcare professional injects Dysport into targeted muscles in the calves (gastrocnemius and soleus) to improve flexibility and movement for children with spasticity affecting the lower limbs.
Contraindications and Precautions

While Dysport can be a valuable treatment for certain conditions, patients and doctors should avoid it in some situations. Understanding these contraindications and taking precautions before prescribing Dysport ensures patient safety and treatment efficacy.
Contraindications for Dysport
- Hypersensitivity: Individuals with a known allergy to abobotulinumtoxinA (Dysport’s active ingredient) or any of its inactive ingredients should not receive Dysport.
- Infection at Injection Site: The presence of an active infection at the intended injection site increases the risk of complications. An aesthetic specialist should postpone treatment with Dysport until the infection clears.
- Allergy to Cow’s Milk Protein: Patients allergic to this ingredient should avoid taking Dysport, as it can trigger a severe reaction, such as breathing difficulties.
- Myasthenia Gravis or Lambert-Eaton Syndrome: These are neuromuscular disorders characterized by muscle weakness. Dysport can worsen these conditions.
Preventive Measures to Be Taken Before Prescribing Dysport
Before prescribing Dysport, a healthcare professional will thoroughly evaluate it to determine if it suits the patient. This evaluation may involve the following:
- Medical History: It is crucial to disclose past medical conditions, such as bleeding disorders, respiratory problems, swallowing difficulties, or pre-existing nerve or muscle weakness.
- Current Medications: Patients must share a complete list of medications they are currently taking, including prescription drugs, over-the-counter medications, and herbal supplements. Certain medications can interact with Dysport, affecting its efficacy or safety.
- Pregnancy and Breastfeeding: Doctors do not recommend Dysport for pregnant or breastfeeding women due to a lack of sufficient safety data.
- Planned Surgeries: Patients should also remember to inform the healthcare professional about any upcoming surgeries, as they may need to consider Dysport’s effects on muscle relaxation.
- Proper Storage and Handling: Healthcare professionals should properly handle and store Dysport and remember to use it within a specific time frame after reconstitution. They must also avoid freezing the formulation.
Dosage and Administration Guidelines
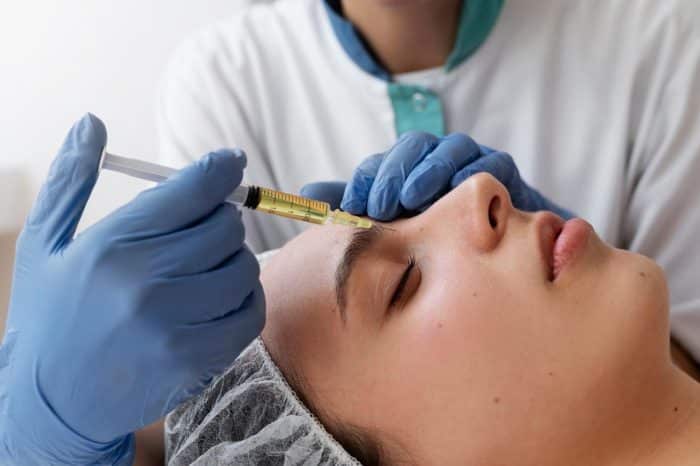
The recommended dosage of Dysport varies depending on the indication and the patient’s needs:
- For adult spasticity, the maximum recommended total dose (upper and lower limb combined) is 1500 Units. A professional divides 500 and 1000 Units among selected muscles at a treatment session.
- In treating cervical dystonia, dosages are tailored to the individual patient based on the location and severity of the muscle spasticity.
- The FDA-approved dose for cosmetic purposes is 50 Units divided into five equal aliquots of 10 Units each.
There are general dosage ranges for spasticity treatment, but it’s important to note that these are for informational purposes only. After a thorough evaluation, a qualified healthcare professional will determine the actual dose.
Administration Techniques and Injection Sites for Optimal Results
Dysport should be administered via intramuscular injection by a qualified healthcare professional. For upper limb spasticity, standard muscles affected include the brachialis, brachioradialis, biceps brachii, and pronator teres.
The recommended dose range in Dysport Units and the number of injection sites per muscle vary, with no more than 1 mL generally administered at any single injection site.
For cosmetic applications, providers using the conventional injection method usually use three to five points.
- One in the middle of the procerus muscle at the mid-brow region
- Two, with one on each side right over the medial canthus, 1 cm over the orbital ridge
- Two in the mid-pupillary line
The dilution of Dysport and the preparation of the solution for administration should use an aseptic technique, and doctors should only administer the reconstituted Dysport through intramuscular injection. The desired final concentration after dilution varies depending on the indication, and the healthcare provider should follow the dilution instructions provided in the Dysport dosing guide.
Patient Selection Criteria
When considering a patient for Dysport treatment, healthcare providers should evaluate the following factors:
- Skin Types: Understanding the patient’s skin type is essential, as certain skin conditions may affect the treatment’s outcome.
- Anatomical Considerations: A healthcare professional will assess the patient’s facial anatomy or the specific muscle groups involved in spasticity for cosmetic and therapeutic uses to determine if Dysport is an appropriate option.
- Medical History: A thorough review of the patient’s medical history is necessary to identify any contraindications or potential risks associated with Dysport use.
- Expectations: It is essential to set realistic expectations with the patient regarding the results of Dysport treatment. Clear communication about what the treatment can and cannot achieve will help ensure patient satisfaction.
- Exploring Other Treatment Options: Once the healthcare professional determines the patient’s goals, they can explore more options. Patients can choose from Dysport or Xeomin, or other similar treatments that align with their aesthetic desires.
- Provider Qualification: Ensuring that the provider is qualified and experienced in administering Dysport is vital for patient safety and optimal results.
Safety Considerations and Adverse Reactions
Dysport, like all treatments involving botulinum toxin, carries potential risks and complications. Common side effects include headaches, fatigue, and injection site reactions such as redness, swelling, and bruising. Rare but more severe complications include muscle weakness, vision changes, and difficulty swallowing.
Healthcare providers can follow several strategies to minimize the risk of adverse reactions and ensure patient safety:
- Educate Patients: Inform patients about the potential risks and signs of complications.
- Proper Technique: Use precise injection techniques and follow the recommended dosages to reduce the likelihood of complications.
- Patient Screening: Carefully screen patients for contraindications and allergies to Dysport components.
- Post-Treatment Monitoring: Monitor patients after treatment for any signs of adverse reactions, especially those related to breathing and swallowing.
- Report and Manage Adverse Events: Encourage patients to report and manage any adverse events promptly to prevent escalation.
Conclusion
Understanding the prescribing information for Dysport is essential for medical professionals to ensure safe and effective use in clinical practice. Medical experts can confidently and responsibly incorporate Dysport into their treatment protocols by considering the indications, contraindications, dosage, and administration guidelines while prioritizing patient safety and satisfaction.
Adhering to the guidelines and best practices for Dysport administration can help optimize treatment outcomes and enhance patient care.
About: DoctorMedica is your trusted supplier of top-quality dermal fillers, viscosupplements, and more for your medical practice. If you’re looking to order Dysport online for your practice, the sales representatives at Doctor Medica can give you guidance.
FAQs
1. How long does it take for Dysport to work?
Dysport typically begins to take effect within 2 to 3 days after treatment.
2. Is Dysport a safe treatment?
Dysport is safe when administered by a qualified healthcare professional as it has FDA approval. However, it can have serious side effects, including difficulties with swallowing and breathing.
3. Who is a good candidate for cosmetic enhancements using Dysport?
A good candidate for Dysport injections is someone with dynamic wrinkles who is in good health and has realistic expectations about the cosmetic results.
References
RealSelf 2022 Most Worth It Honorees. RealSelf.com. https://www.realself.com/most-worth-it
Galderma. A Decade of Dysport®: Galderma Celebrates 10 Years Since FDA Approval of Dysport in the U.S. Prnewswire.com. Published April 30, 2019. https://www.prnewswire.com/news-releases/a-decade-of-dysport-galderma-celebrates-10-years-since-fda-approval-of-dysport-in-the-us-300840790.html
Molina B, Grangier Y, Môle B, et al. Patient satisfaction after the treatment of glabellar lines with Botulinum toxin type A (Speywood Unit): a multi‐centre European observational study. Journal of the European Academy of Dermatology and Venereology. 2014;29(7):1382-1388. doi:10.1111/jdv.12881
Related Articles
Joanna Carr
Botox For Sweaty Hands
Interested to learn more about Botox For Treatment Of Sweaty Hands? Browse Doctor Medica's comprehensive listing of blog posts.
Joanna Carr
Juvederm for Neck: Rejuvenation with Hyaluronic Acid Filler
Juvederm fillers offer a non-surgical solution for neck rejuvenation, addressing skin laxity, horizontal lines, and loss of definition.
Joanna Carr
Cytocare 532 Before and After Including Photos
Explore the transformative effects of Cytocare 532 with before-and-after photos. Learn how this treatment rejuvenates skin, reduces wrinkles, and boos...


