Steroid medications, particularly corticosteroids, are often used to manage arthritis pain effectively; however, recent research has highlighted significant risks associated with their use. A compelling University of Michigan study revealed that even brief steroid use could double the risk of blood clots and bone fractures within three months, while increasing hospitalizations for sepsis fivefold within just 30 days.
In contrast, Euflexxa is an injectable therapy derived from hyaluronic acid (HA), specifically formulated to mimic the natural fluid that lubricates and cushions knee joints affected by osteoarthritis. Unlike steroid injections, which primarily target inflammation, Euflexxa focuses on restoring joint lubrication and mobility without the systemic risks typically associated with steroid treatments.
This article will clearly explain what Euflexxa is, explore how it fundamentally differs from steroid injections, and help clarify whether it is a suitable alternative for those questioning, “Is Euflexxa a steroid?”
Key Takeaways
- Euflexxa is not a steroid; it is a Class III medical device regulated by the FDA, specifically a hyaluronic acid-based viscosupplement that treats knee osteoarthritis through combined mechanical and biochemical actions.
- Unlike corticosteroids, Euflexxa provides joint relief primarily by enhancing lubrication, cushioning, and restoring the viscosity of natural synovial fluid, rather than by suppressing inflammation chemically.
- Recent studies have recognized that Euflexxa also offers significant biochemical benefits, including the reduction of inflammatory mediators and the potential protection of joint cartilage health over time.
- Euflexxa has a favorable long-term safety profile, free from systemic side effects commonly associated with repeated steroid injections, including cartilage thinning and hormonal disruptions.
- Clinicians often adopt a combined treatment strategy, initially using corticosteroids for rapid symptom control, followed by a transition to Euflexxa for sustained joint management and long-term protection.
About: Doctor Medica is your trusted supplier of top-quality dermal fillers, viscosupplements, and more for your medical practice. We offer genuine products from leading brands at the lowest prices in the market. If you’re looking to order Euflexxa online for your practice, contact Doctor Medica today.
Product Classification & Clinical Differentiation
When evaluating treatment options for knee osteoarthritis (OA), it’s crucial to differentiate clearly between products like Euflexxa and traditional steroid injections, not only regarding their mechanisms of action but also their regulatory classification. Patients frequently ask if viscosupplements such as Euflexxa are considered drugs, and this distinction affects prescription practices and anticipated side effects.
Contrary to corticosteroids, Euflexxa is not a pharmaceutical drug. In the United States, both Euflexxa and Synvisc are classified as Class III medical devices, subject to rigorous premarket approval (PMA) by the FDA under 21 CFR 888.3569 and PMA P010029.
Similarly, in the European Union, injectable hyaluronic acid (HA) products like Euflexxa and Synvisc also fall under the strict regulatory oversight of the Medical Device Regulation (MDR), classified as Class III devices due to their invasive use and risk profile (Annex VIII, Rules 8–9).
Comparing Euflexxa to Other HA-Based Injections & Corticosteroids
| Feature | Euflexxa | Synvisc | Corticosteroids (e.g., Kenalog) |
| Classification | Class II medical device | Class III medical device | Pharmaceutical drug |
| Active Ingredient | Sodium hyaluronate (non-avian origin) | Hylan G-F 20 (avian-derived, cross-linked HA) | Triamcinolone, methylprednisolone, etc. |
| Mechanism of Action | Mechanical lubrication, shock absorption | Mechanical + some anti-inflammatory potential | Suppresses immune/inflammatory response |
| Steroid Content | None | None | Yes |
| Onset of Relief | Gradual (weeks) | Moderate (often faster than Euflexxa) | Rapid (within days) |
| Regulatory Oversight | FDA-cleared device | FDA-cleared device | FDA-approved drug |
As the Euflexxa vs Synvisc comparison shows, although both are steroid-free viscosupplements, they differ significantly in molecular origin, cross-linking, and biochemical interactions within the joint.
Euflexxa, derived from non-avian sources, typically carries a lower allergy risk, while Synvisc might provide quicker relief but higher immunogenic potential. Corticosteroids, while helpful for immediate inflammatory relief, have limited suitability for prolonged treatment due to systemic risks.
Understanding these classifications and regulatory contexts, particularly in light of the ongoing FDA review initiated in 2018 regarding HA product categorization, enables clinicians to make more informed treatment choices that prioritize patient safety and clinical efficacy.
Mechanism: Mechanical vs Biochemical Action
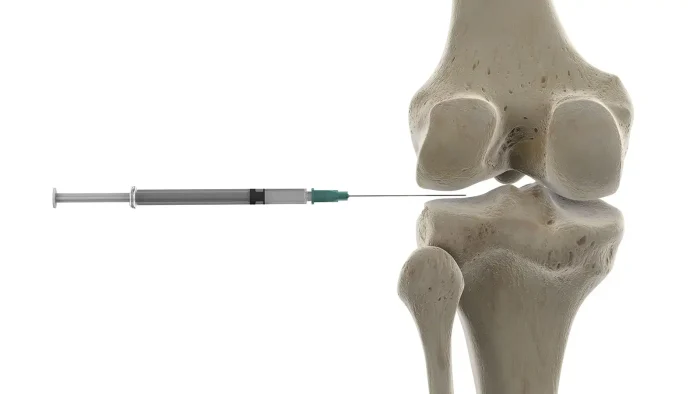
Understanding how Euflexxa functions involves recognizing its dual-action mechanism—both mechanical and biochemical. This combination distinguishes it from corticosteroids, which rely predominantly on biochemical, anti-inflammatory pathways.
Mechanical Action
Primarily, Euflexxa enhances the mechanical properties of joint fluid:
- Improves viscosity and lubrication, closely mimicking the body’s natural synovial fluid.
- Cushions the joint, reducing friction between cartilage surfaces.
- Enhances shock absorption, protecting the joint during movement and physical activity.
By physically restoring joint fluid characteristics, Euflexxa provides immediate mechanical support, aiding smoother, less painful joint movement without systemic immunosuppressive effects.
Biochemical Action
Recent research highlights that hyaluronic acid injections, including Euflexxa, also demonstrate significant biochemical effects within the joint:
- Modulates inflammatory pathways, potentially reducing inflammatory mediators within joint tissues.
- Protects cartilage health by promoting chondrocyte viability and reducing cartilage-degrading enzymes.
- Stimulates the joint’s own production of hyaluronic acid, contributing to sustained joint function and comfort over time.
Although initially recognized primarily for its mechanical support, the biochemical contributions of Euflexxa substantially enhance its therapeutic value, making it beneficial for the management of chronic osteoarthritis.
Comparison to Corticosteroids
In contrast, corticosteroids like Kenalog and Depo-Medrol focus mainly on biochemical actions by:
- Rapidly suppressing inflammatory responses.
- Blocking immune cell activation and cytokine release.
- Providing swift relief during acute osteoarthritis flare-ups.
However, corticosteroids do not provide mechanical joint support and may carry significant side effects with repeated use, including cartilage thinning and systemic hormonal imbalances.
Clinical Implications
Clinicians should consider both mechanical and biochemical benefits of Euflexxa when planning treatments. This comprehensive approach may offer:
- Gradual but sustained symptom relief.
- Improved joint protection over time.
- Reduced long-term systemic risks compared to steroid injections.
This balanced mechanism underscores why Euflexxa is often a preferred long-term therapy in the management of knee osteoarthritis.
Safety Profile vs Corticosteroids
Safety remains a critical concern when considering treatments for chronic knee osteoarthritis. While corticosteroids provide immediate anti-inflammatory benefits, frequent use carries documented risks, prompting many clinicians and patients to seek safer long-term alternatives such as Euflexxa.
Cartilage Health
- Corticosteroids: Repeated injections can accelerate cartilage thinning, potentially worsening OA progression.
- Euflexxa: Does not degrade cartilage; research suggests potential protective effects on joint integrity.
Systemic Side Effects
- Corticosteroids: Potential systemic complications like immune suppression, hyperglycemia, adrenal disruption, and bone density reduction.
- Euflexxa: Primarily localized effects, significantly reducing systemic risk.
Injection Frequency and Tissue Health
- Corticosteroids: Generally limited to 3–4 injections annually due to safety concerns (skin thinning, pigment changes, infection risks).
- Euflexxa: Safely administered every six months, aligning with a consistent and safer long-term OA management strategy.
While corticosteroids are valuable for immediate relief, Euflexxa provides a preferable safety profile for long-term joint management.
Treatment Strategy and Patient Implications
Choosing between Euflexxa and corticosteroids ultimately hinges on individual patient needs, clinical severity, and treatment objectives. Corticosteroids may be appropriate for rapid inflammation reduction during acute OA flares, whereas Euflexxa is suited to ongoing management, protecting joint health without systemic adverse effects.
A combined treatment approach is often employed: corticosteroids initially to rapidly control severe symptoms, followed by transitioning to Euflexxa for longer-term maintenance therapy. Such a dual-phase strategy helps balance immediate symptom relief and sustained joint function improvement, promoting lasting mobility and quality of life.
Clinicians and patients should jointly discuss:
- Symptom urgency vs. long-term health considerations.
- Allergy profiles (favoring Euflexxa’s non-avian composition).
- Scheduling constraints and preferred treatment frequency.
Conclusion
Euflexxa fundamentally differs from steroid injections, offering a safe, steroid-free, and regulatory-approved (Class III medical device) treatment for knee osteoarthritis. Recognizing clearly that Euflexxa is not a steroid but a viscosupplement with mechanical and biochemical joint-protective actions profoundly impacts patient and clinician decision-making, ensuring safe, effective, and personalized osteoarthritis management.
For optimal treatment planning, patients should discuss with their healthcare providers whether Euflexxa, corticosteroids, or a thoughtfully combined therapeutic strategy aligns best with their individual OA management goals.
FAQs
1. Does Euflexxa reduce inflammation like steroids?
No, Euflexxa works mechanically by moisturizing and cushioning the joint. It doesn’t chemically suppress inflammation like corticosteroids do.
2. How often can I get Euflexxa injections?
Following the standard Euflexxa dosage, you may receive three injections one week apart and repeat the series every six months as needed.
3. Can I have steroid and Euflexxa injections together?
Yes—some doctors use a steroid injection for immediate pain relief, followed by Euflexxa to maintain joint health. Discuss timing and safety with your provider.
4. Are there side effects from Euflexxa?
Mild joint swelling, pain, or warmth at the injection site may occur, but systemic side effects common with steroids are not expected.
References
Waljee AK, Rogers M a M, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ. Published online April 12, 2017:j1415. doi:10.1136/bmj.j1415
Gavin K. Common drugs, uncommon risks? New study looks at serious health problems after Short-Term Steroid use. Published April 13, 2017. https://www.michiganmedicine.org/health-lab/common-drugs-uncommon-risks-new-study-looks-serious-health-problems-after-short-term
Related Articles
Joanna Carr
Jaydess Reviews – Is The Contraceptive Good?
Jaydess is a hormonal intrauterine device (IUD) that provides up to three years of continuous birth control by releasing a low dose of levonorgestrel ...
Joanna Carr
Sculptra Hips – Minimally Invasive Volume Building
Learn about Sculptra for hip enhancement, a minimally invasive treatment option for adding volume and achieving a more contoured silhouette.
Joanna Carr
Plastic Surgery As A Status Symbol
Interested in learning more about Plastic Surgery As A Status Symbol? Browse Doctor Medica's comprehensive archive of blog posts.


