FDA approval is a critical benchmark in the medical and cosmetic industries, ensuring that products meet stringent safety, efficacy, and quality standards. In 2022 alone, the FDA approved 37 novel medical products, reflecting the importance of rigorous testing and regulatory oversight.
Neuramis, a hyaluronic acid-based dermal filler, has gained significant attention for its innovative SHAPE technology and versatile applications. Widely used in aesthetic treatments such as wrinkle reduction and lip enhancement, Neuramis is popular in many countries. However, its FDA approval status remains a frequent topic of inquiry among potential users.
This article will examine Neuramis’s FDA approval status, its safety profile, and what it means for those considering it for cosmetic procedures.
Key Takeaways
- Neuramis uses advanced SHAPE technology, incorporating hyaluronic acid and cross-linking methods for long-lasting and natural results.
- FDA approval ensures compliance with stringent safety and efficacy standards, reassuring practitioners and patients.
- Neuramis is approved for its safety and effectiveness in Europe and parts of Asia, such as Thailand. However, it has not yet received FDA approval in the United States.
- Regulatory processes vary by region. Europe focuses on safety and device performance, while the U.S. emphasizes both safety and effectiveness.
- Without FDA approval, Neuramis cannot be legally marketed in the U.S., requiring practitioners to rely on FDA-approved alternatives while informing patients about these regulatory considerations.
About: Doctor Medica is your trusted supplier of top-quality dermal fillers, viscosupplements, and more for your medical practice. We offer genuine products from leading brands at the lowest prices. Contact Doctor Medica today to order Neuramis online for your practice.
Understanding Neuramis Filler

Neuramis is a hyaluronic acid-based dermal filler designed for a variety of aesthetic applications, including smoothing wrinkles, restoring facial volume, and enhancing lips. Developed using SHAPE technology, Neuramis fillers offer a high degree of purity and stability, ensuring natural-looking and long-lasting results.
Key components of Neuramis include:
- Hyaluronic Acid: A naturally occurring substance that hydrates and plumps the skin.
- Cross-Linking Technology: Enhances the filler’s longevity and effectiveness.
The importance of FDA approval for medical products like Neuramis cannot be overstated. FDA approval serves as a critical benchmark, signifying that a product has undergone rigorous testing to ensure its safety, efficacy, and quality. For patients and practitioners alike, this approval provides an added layer of trust and confidence in the product’s reliability.
Neuramis Filler’s Regulatory History

Neuramis filler has had a varied regulatory journey across different regions. In Europe, Neuramis has received approval from the European Directorate for the Quality of Medicines and HealthCare (EDQM), making it widely available and considered safe for use. Similarly, the Thai FDA has also approved Neuramis in Asia, allowing its use in aesthetic treatments.
However, Neuramis has not yet received FDA approval in the United States. This means it is currently unavailable for cosmetic treatments in the U.S., and practitioners must inform patients about potential risks and consider FDA-approved alternatives.
The differences in regulatory processes across these regions highlight the varying standards and requirements for medical products. Europe tends to focus more on safety and device performance, while the U.S. emphasizes both safety and effectiveness. This can lead to faster approvals in Europe but also raises concerns about the sufficiency of evidence for clinical benefits
Implications for Medical Practitioners
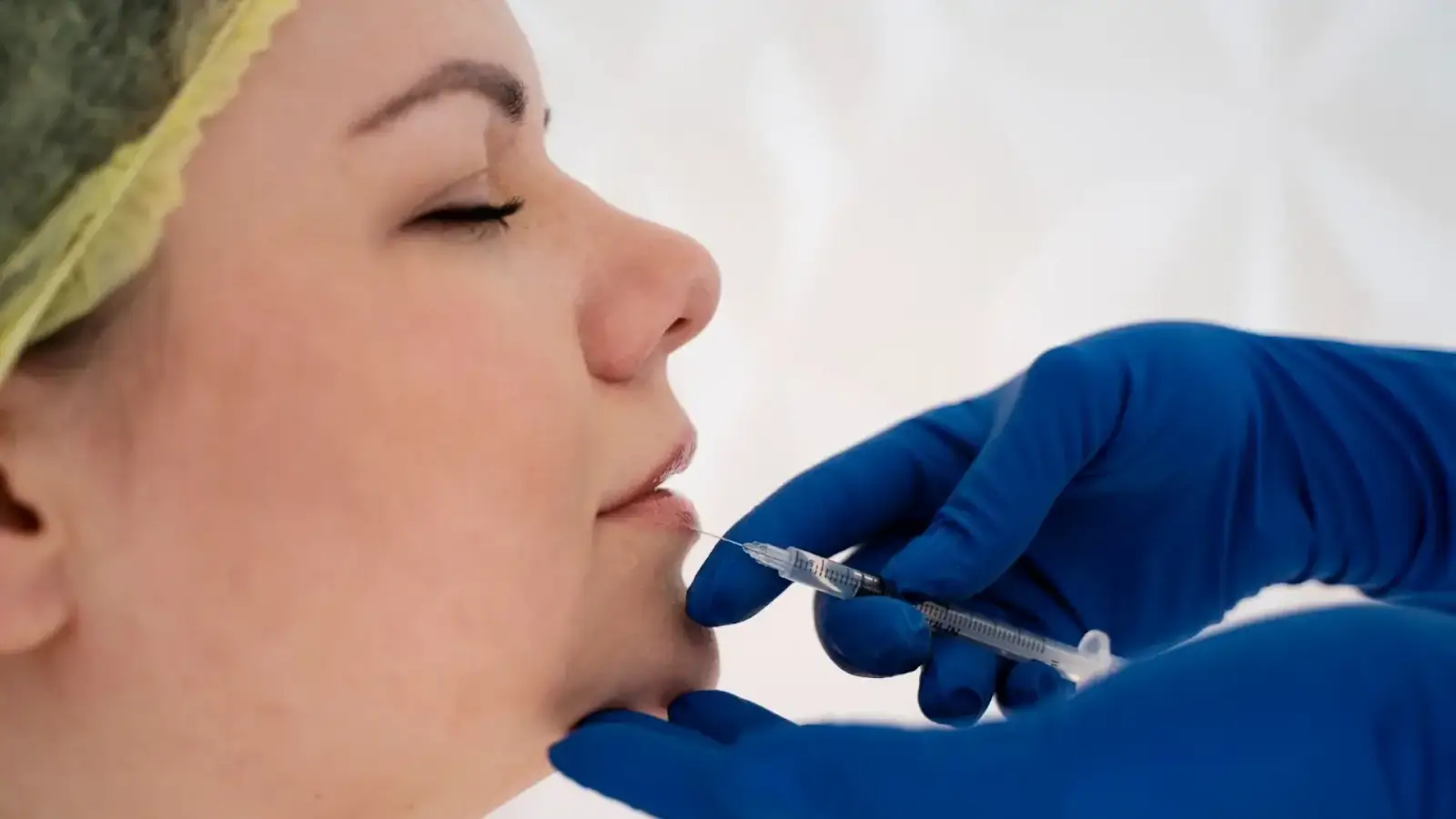
For medical practitioners, FDA approval of a product like Neuramis filler has significant implications. Legally, using an FDA-approved product ensures compliance with stringent regulatory requirements, minimizing the risk of liability issues.
It also provides a benchmark for safety and efficacy, giving practitioners confidence in the product’s quality and performance. Additionally, FDA approval facilitates easier integration into clinical practice, as the product adheres to established medical standards.
Compliance with local regulations is crucial. Practitioners must ensure they are not only using approved products but also adhering to specific guidelines for their use. This involves staying updated on any changes in regulatory status and understanding the differences in approval processes across regions.
Aside from Neuramis filler before and after results, approval from regulatory bodies like the FDA reassures patients and practitioners of the product’s credibility and effectiveness. Patients are more likely to feel secure in their treatment choices, knowing that the product has passed rigorous testing.
Conclusion
Neuramis fillers have gained widespread approval and trust across Europe and Asia for their innovative SHAPE technology and proven effectiveness. While their FDA approval is pending, the product remains a popular choice in markets where it is authorized, delivering reliable and natural-looking results.
For U.S.-based practitioners and patients, FDA-approved alternatives are necessary until Neuramis completes the regulatory process. Understanding these nuances ensures informed decisions and safe applications in aesthetic medicine.
FAQs
1. How does Neuramis compare to FDA-approved fillers?
Thanks to its SHAPE technology, Neuramis offers similar benefits, such as natural-looking results and longevity. However, its lack of FDA approval limits its availability in the U.S.
2. Why is FDA approval important?
FDA approval guarantees that a product has met rigorous testing for safety, efficacy, and quality, providing patients and practitioners with confidence in its use.
3. How can practitioners stay updated on Neuramis’s approval status?
Practitioners should monitor updates from regulatory bodies and consult reputable sources to stay informed about changes in Neuramis’s FDA approval status.
References
Food and Drug Administration. (2022). FDA annual report 2022 (Final version). Food and Drug Administration Philippines. Retrieved from https://www.fda.gov.ph/wp-content/uploads/2023/05/FDA-Annual-Report-2022-Final.pdf
Medytox Academy. (n.d.). Neuramis. Medytox Academy. Retrieved December 2, 2024, from http://medytoxacademy.com/page/neuramis
Related Articles
Joanna Carr
VOM Filler For Lips – About VOM O
Learn about VOM Filler for lips, specifically VOM O—explore its features, benefits, and how it enhances lip volume and definition naturally.
Joanna Carr
Botox Reconstitution Guide
Learn everything about Botox reconstitution: dilution ratios, step-by-step guide, and tips for medical professionals to achieve optimal results.
Joanna Carr
Supartz vs Euflexxa – Which is Better?
Supartz is purified sodium hyaluronate, while Euflexxa is composed of highly purified, partially cross-linked sodium hyaluronate.


